
A bottleneck in drug discovery has grown from a reliance on traditional Target ID approaches. There is a huge backlog of undruggable targets, as well as a huge amount of potential in undrugged targets. Last October at Oxford Global’s Discovery UK: In-Person event, our Chief Technology Officer, Dr Benedict Cross, described PhoreMost’s current focus on ‘Drugging the Undruggable ®’. By focusing on different targets and pathways, we are taking a systematic approach to identifying novel drug targets and finding druggable/cryptic sites on known targets previously thought to be undruggable:
Target Identification: Expanding the Known Druggable Space
“We know there’s an untapped drug space,” began Ben, “and much of it is known drug targets which have proven themselves undruggable.” The extant genetic strategies for Target ID gravitate towards druggable spaces – so the research and development of drugs is focused on known druggable targets in the human proteome. Known members of a druggable class are favoured since drug screening campaigns are expensive, and there are few technologies available when it comes to identifying new targets. As Ben said, “It’s difficult to break out of this known druggable space.”
"What we need to escape this undruggable whirlpool is a technology that discovers a target and delivers a mechanism to drug it."
Part of the issue is down to the sensible series of pragmatic steps that each treatment has to follow. Drug discovery scientists are unlikely to go through with the risks associated with taking unknown targets to a new space when known targets have already been subject to years of prior research. “What’s needed to escape this whirlpool is a technology that discovers a target while at the same time delivering a mechanism to drug that target,” Ben continued. PhoreMost is working to address this need by building a pipeline of novel drug discovery programmes aimed at addressing a range of unmet diseases.
Drug Development for the RNA Delivery Landscape
One solution we’ve championed is PROTEINi, a screening operation that exploits target proteins. “When I say PROTEINi, it describes a relatively simple concept,” he said, “which is the use of peptide fragments in place of small molecules.” These pools of programmed mini-proteins are used in place of small molecules to explore the biological nodes responsible for a given disease phenotype (as seen in Figure 1). This new technology enables the discovery of new targets and crucially, identifies the pockets into which drugs can be designed. “What we’re looking to explore here is the presentation of drug-like molecules in a physiological system that you can use to monitor and interfere with disease progression,” clarified Ben.
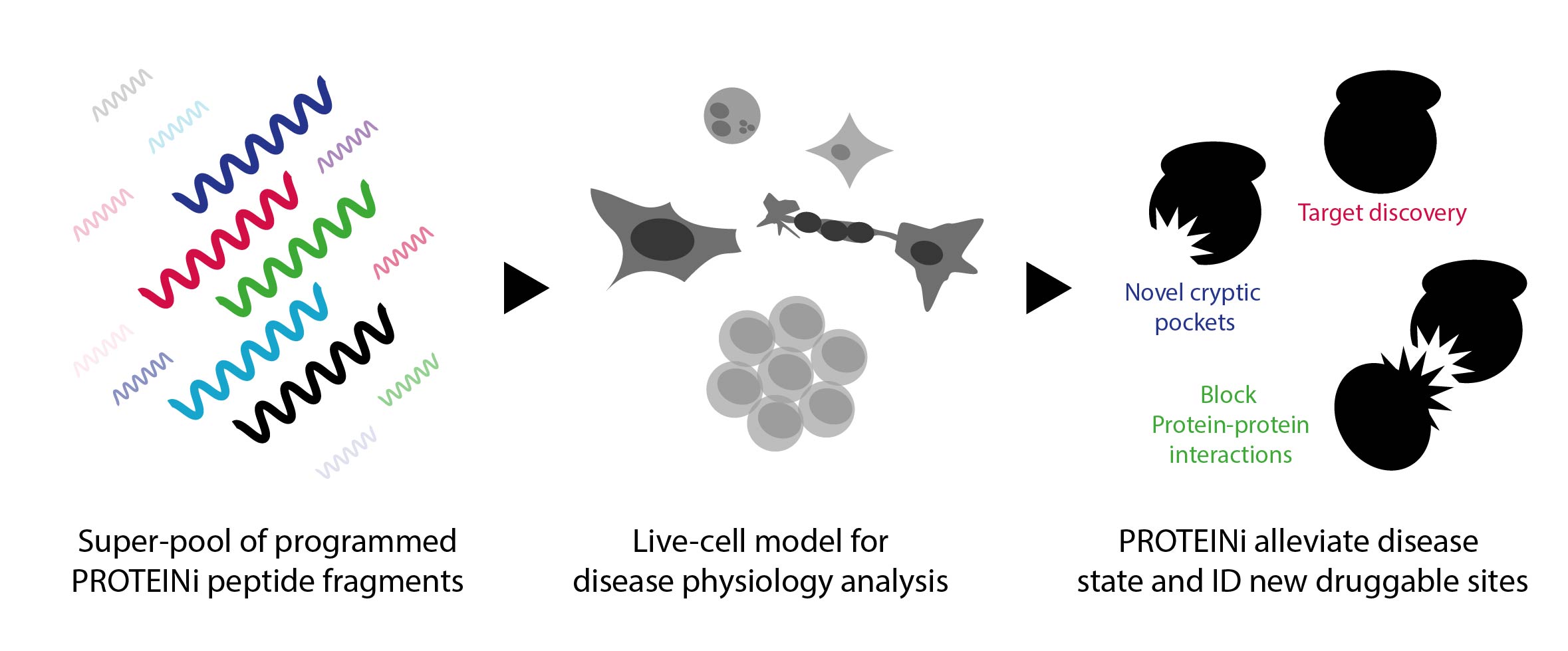
Figure. 1 – The process of identification and discovery using PROTEINi peptide fragments.
“Strategically speaking, what we’re looking to uncover in this is not necessarily a peptide which is active, but a peptide which describes a new starting point for drug discovery. However, the past two years have seen the RNA delivery landscape change dramatically, especially with the successful use of virus and lipid nanoparticle vehicles for COVID19 vaccines”.
PhoreMost’s phenotypic screening platform, SITESEEKER®, allows for the direct discovery of mRNA-encodable, intracellularly active mini-proteins (PROTEINi), which are ideal inputs into these new therapeutic delivery technologies. In one example discovery campaign Ben described, we investigated novel synthetic lethal gene interactions for the selective death of certain cell types, e.g. in cancer. The route for development can therefore be multipronged, either by direct drug development where targets are structurally-enabled, or via novel modalities like targeted protein degradation (TPD) or mRNA delivery approaches.
Target Identification: Warheads and Novel E3 Binders
The opportunities for the application of the technologies developed by PhoreMost are sizable, particularly when it comes to TPD. “We’re focusing predominantly on oncology,” continued Ben, “and more recently we’re really interested in targeted protein degradation.” He added that he was excited to talk about the development of new TPD-based drugs and the opportunity to couple PhoreMost’s warhead discovery approach with novel E3 ligase binders to create heterobifunctional drugs. “Our platform can uncover phenotypically active warheads, but they can also be exploited in a TPD strategy, and we have also identified numerous new binders to E3 ligases” he explained.
Planning the Future of Drug Development
Rounding off his presentation, Ben explained that the positioning of our platform in capitalising on the new opportunities in induced proximity is very timely, as these new and exciting modalities are starting to reach clinical validation. At PhoreMost, we are continually developing and refining our technology. As he described, the continued focus for PhoreMost will be on the discovery of new ligases and drug molecules in core therapeutic areas, and that there are opportunities for partnerships that could maximise the use of our platform. “We have bold ambitions to go even further than we have done so far,” he concluded. “We are configuring this approach to try and counter some of the bottlenecks in drug discovery, in discovering new ligases and new degrader molecules.” By expanding the druggable toolbox and improving access to undruggable targets, PhoreMost is aiming to do just that.
This was originally transcribed by Ben Norris at Oxford Global and can also be found published here.
For more information on events by Oxford Global.